Certificates & Compliance.
Lifecom Pharmaceuticals prioritizes regulatory compliance and superior quality in every therapeutic drug, bulk pellet, skincare, health, and nutrition product we manufacture and market.
Our certifications reflect 25+ years of dedication as a world-class healthcare provider and the fastest-growing pharma company in India.
GMP Certificate
Validates Good Manufacturing Practices for pharma standards.








25th Anniversary Certificate
Commemorating 25 years of pharmaceutical excellence, innovation, and healthcare impact since Lifecom Pharmaceuticals' founding in 2000.
WHO-GLP Certificate
World Health Organization Good Laboratory Practices validating reliable, reproducible non-clinical research data for regulatory submissions.
WHO-GMP Certificate
Lifecom's facilities meet international standards for pharmaceutical production, covering everything from raw materials to final packaging.
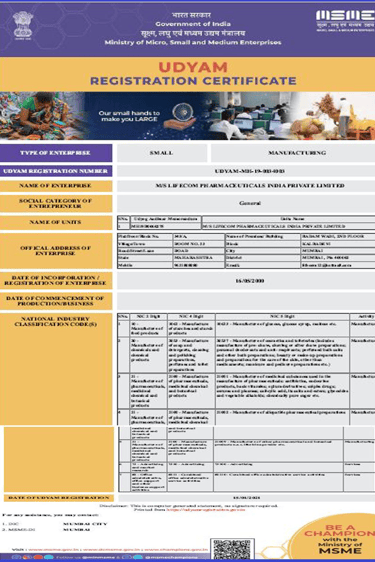
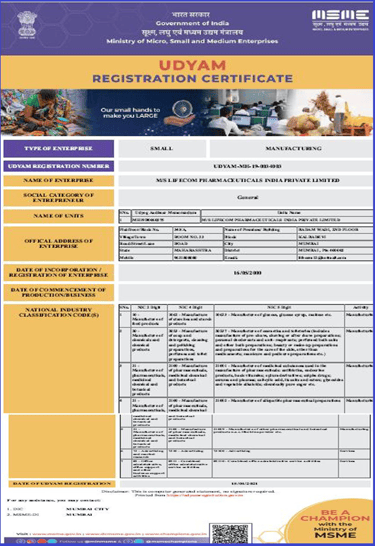
UDYAM Certificate
Government of India Micro, Small & Medium Enterprises registration, enabling priority sector benefits and market access.


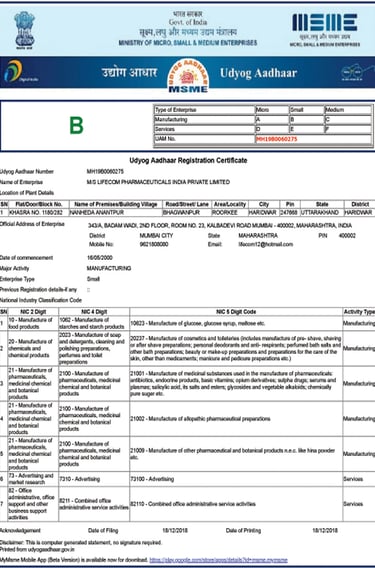
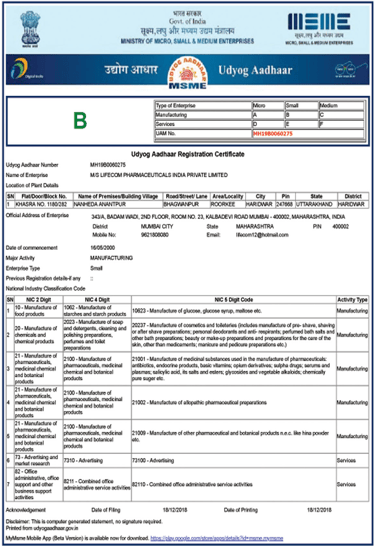
MSME Certificate
National single-window MSME identification for streamlined government tenders, subsidies, and procurement preferences.
ISO 9001:2015 Certificate
Global Quality Management System certification guarantees customer satisfaction and continuous improvement processes
What certifications does Lifecom Pharmaceuticals hold?
Key ones include WHO-GMP, ISO 9001:2015, ISO 22000:2018, and GMP for cosmetics.
Is Lifecom GMP certified for pharma manufacturing?
Yes, WHO-GMP certified for therapeutic drugs and bulk pellets production in India.
What does WHO-GMP certification mean for Lifecom?
It confirms international manufacturing standards for safe, quality pharmaceutical production.
Is Lifecom ISO 9001:2015 certified?
Yes, ensuring comprehensive quality management across all operations.
How current are Lifecom's certificates?
All certifications are valid and renewed per regulatory cycles—verify via downloads.
Does Lifecom have FSSAI certification?
Yes, for health and nutrition products under ISO 22000 and FSSAI compliance.
What is UDYAM registration for pharma companies?
India's official MSME identification enables government benefits and tenders.
How current are Lifecom's certifications?
All certificates are actively maintained with annual renewals and audits.
Frequently Asked Questions (FAQs)
Registered HQ
316, Hind Nagar, Kanpur Road, Lucknow, Uttar Pradesh 226012, IN
(+91) 0522 406 6541
+91-9876543210
Lifecom Pharmaceuticals (I) Private Limited.
Industrial
Khasra No. 1180/282 Nanhera Anantpur, Bhagwanpur, Roorkee District, Haridwar Roorkee, Uttarakhand, IN
(+91) 9258152056/57/61, 92582 92721, 9258293990
Making your Life Comfortable.
© 2026 All rights reserved.

